Understanding Colloids, Colloidal Nanoparticles & Silver Colloids
This short primer on colloidal particles is really just a series of notes; hopefully, they give you a basic understanding of colloids in general, and silver colloids specifically. Let’s begin by taking a look at particle size.
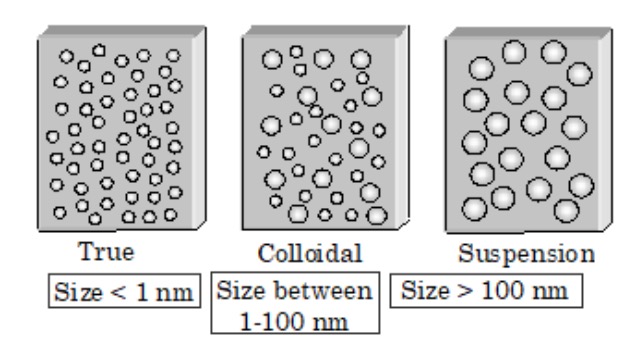
- If the particle size is greater than 500 nm, then we have a suspension – similar to the micelles in homogenized milk.
- If the particle size is between 2 nm and 500 nm, we have a colloid – the particles are small enough to maintain an even dispersion throughout the solvent. They, essentially, remain suspended. Gelatin (jello), smoke and fog are colloids. Investigate the particle sizes specifically in these.
- If the particle size is less than 2 nm, then you have a solution. Such a tiny size equals a huge (relative) area for interaction. The solute is the thing that dissolves, and the solvent is the thing that’s dissolving.
***Example: Sodium chloride dissolves in water for the following reasons: Na is a cation (positively charged), whereas Cl is an anion (negatively charged). When placed in H20, in which the H is positively charged and the O is negatively charged, the Na (cation) will be attracted to the negatively charged O, and the Cl (anion) will be attracted to the positively charged H – which causes the solution to dissolve. Crucially, the particle size is so tiny that the huge surface area also produces a liberal surface for the interaction.

4. An angstrom is one-tenth of a nanometer (10^-10 m) or 0.1 nm.
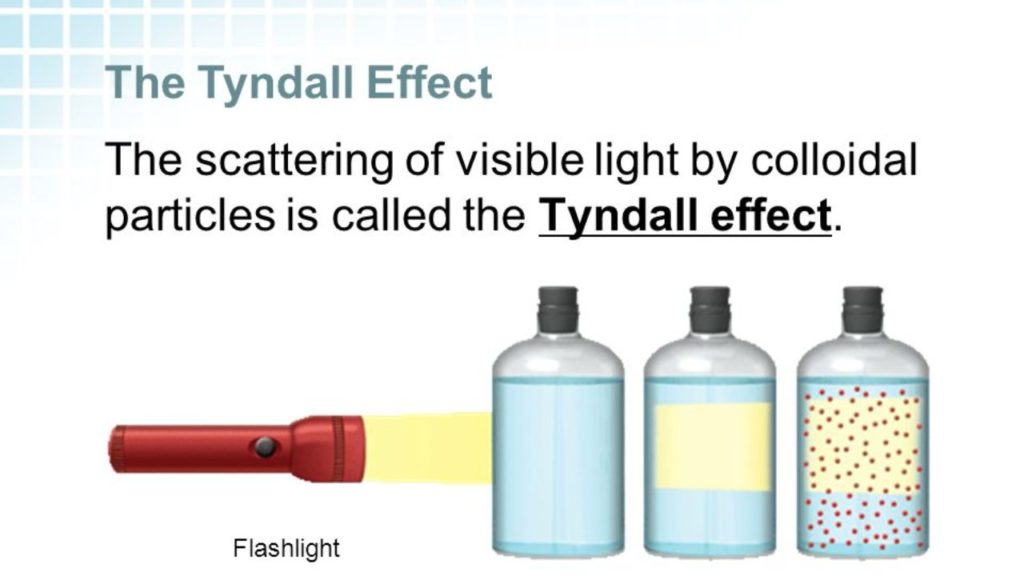
5. The Tyndall Effect is an important characteristic that distinguishes between suspensions, solutions and colloids. This effect describes how light diffracts when it is shone through any of the above mixtures. In a solution, where the particle sizes are less than 2 nm, they are not large enough to scatter light, and so we cannot see the path of light. In a colloid and in a suspension, the particles are big enough to scatter light; it is this scattering that is called the Tyndall Effect. In sum, then:
- In a suspension, the particles are greater than 1000 nm and the Tyndall Effect can be observed. However, since particles of this size cannot maintain an even dispersion throughout the solute, they settle down eventually and the Tyndall Effect disappears. A suspension is a heterogeneous mixture, since the two substances are not in even solution and are visibly separate.
- In a colloid, the particles are less than 500 nm but greater than 1 or 2 nm; these are able to maintain an even dispersion throughout the solvent because of Brownian motion and intermolecular forces. As a result, the Tyndall Effect is always observed in a colloid. The size of the particles themselves must be comparable to the wavelength of the incoming visible light in order for them to be able to scatter said light. Additionally, although colloidal particles can range up to 500 nm-1000 nm, colloidal nanoparticles are generally considered to be between 1 and 100 nm – a size at which they subsume new electrical and mechanical properties.
- In a solution, the particles are smaller than 1 or 2 nm and so there are never particles large enough to scatter light. There is no Tyndall Effect. The tiny size of the particles further exacerbates their penchant to interact liberally with the local environment.
6. A foam is also a colloid – it’s just an example of a gas suspended in a liquid. Consider the different types of colloids, in terms of a solute dispersed throughout a solvent (the one that exists in greater quantity is the solvent or medium):
- Foam – a gas suspended in a liquid (the gas is the dispersive phase and the liquid is the dispersed medium since there’s more of the latter)
- Sol – a solid dispersed in a liquid like a starch solution
- Emulsion – a liquid dispersed in another liquid, as in dilute milk
- Gel – a liquid dispersed in a solid, as in jellies/gelatin
- Aerosol – a solid dispersed in a gas, such as fog, mist or smoke
Colloids are distinguishable, primarily, by two properties: the Tyndall Effect (persistent) and Brownian motion. The Tyndall cone is observable in the case of a colloid, and it remains as such unlike with a suspension (it dies down in the latter after awhile since the particles cannot maintain even dispersion because of their large size).
In colloids, since, although they may be called solutions colloquially, they are not actually that; technically, we need a different name to replace solute and solvent (nothing is dissolving in a colloid, after all). Thus, the dispersive phase is the solute and the dispersed medium is the solvent.
Examples of colloidal solutions: blood, honey, milk, toothpaste, gum. Aerosols – gas, mist or smoke; solid or liquid dispersed in a gas (the solid/liquid is the dispersive phase and the gas is the dispersive medium.
7. The Amazon River is a colloid; the brown river is full of sediments that comprise a suspension; if you take a glass cup of this river, these large sedimentary particles will fall to the ground after some time (as befits a true suspension).

However, the water will still be brown because there are microscopic clay particles in it; this is now a colloid because these clay particles are able to maintain an even dispersion throughout the water. The size range must be between 1 nm and 500 nm or so. Recall that nanoparticles are generally considered to be less than 100 nm but more than 1 or 2 nm. The clay minerals are being bombarded by water molecules as delineated by Brownian motion.
Why don’t the colloidal clay minerals stick together when they collide, since the water molecules are smashing them together so often? Because the clay colloidal dispersive phase carries positive charge, and like charges repel. Now, when the river reaches the saltwater ocean, the NaCL, with Na positive and CL negative, something happens: the Cl- is preferentially attracted to the positively charged colloidal clay minerals, which lessens the repulsive effect. Essentially the repulsive electric field of the clay minerals is attenuated by the salt cloud and the tiny clay particles can now clump together – which causes them to grow larger and precipitate out of the realm where Brownian motion can keep them in even dispersion throughout the dispersion medium.
The above is what is responsible for river deltas – when colloidal freshwater from river tributaries meet the salty ocean water, they can form complex islets of minerals. The cascading rivulets below, in the mighty Ganges River of India, is yet another example of a colloidal phase that transitions into a suspension as the particles clump together and precipitate out of the colloid. They, essentially, become too large for Brownian motion to sustain their even dispersion.

8. At high concentrations, colloidal particles (the dispersive phase within a dispersive medium) can form a liquid. At even higher concentrations, they can form a crystalline structure – the Brownian motion is always there, though. How then, do the resulting substances still “flow”, or are still soft? This is actually a function of the distance between the particles vs the particle size. As long as the average distance between the particles is much larger than their size (for colloids, the size is between 1 nm and 1000 nm (1 micrometer), then they possess the fore-mentioned characteristics.
9. Coffee is also a colloid – perhaps surprisingly. It shouldn’t be that surprising, however, given that the color remains brown no matter how long the coffee sits. This because the coffee particles are smaller than a micrometer. Why is coffee not a solution, then? All you have to do is test it to discern the Tyndall Effect. If you can see the path of the light through it, then it is a colloid and won’t settle its constituent parts a la a suspension.

10. Artificial rain is also caused by taking advantage of colloidal properties. Clouds possess colloidal water particles that maintain an even dispersion throughout the cloud. By using a high altitude plane to sprinkle oppositely-charged colloidal dust/sand particles over these clouds, we can neutralize the like charge that the colloidal water particles use to repel each other. As a result, they coagulate and fall as rain, and thus have effectively become a suspension.

